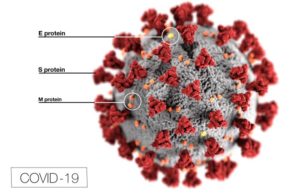
COVID-19 illustration by the Centers for Disease Control and Prevention.
WASHINGTON (AP) — U.S. government advisers will debate Friday if there’s enough proof that a booster dose of Pfizer’s COVID-19 vaccine is safe and effective.
It’s the first public step toward deciding which Americans may get an extra dose and when.
The Food and Drug Administration on Wednesday posted much of the evidence that it will ask outside experts to consider at Friday’s meeting.
But the agency struck a cautious tone in reviewing the data and discussing the rationale for boosters.
That careful approach is notable given that White House officials have been previewing a booster campaign that they hoped to begin next week.
| By LAURAN NEERGAARD and MATTHEW PERRONE, The Associated Press |