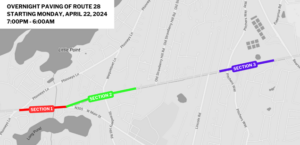
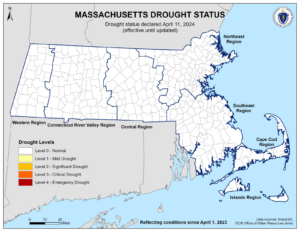
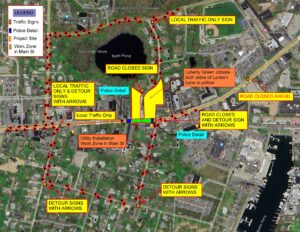
WASHINGTON (AP) – U.S. federal health officials say results from a U.S. trial of AstraZeneca’s COVID-19 vaccine may have used “outdated information.”
The Data and Safety Monitoring Board said in a statement that it was concerned that AstraZeneca may have provided an incomplete view of the efficacy data.
AstraZeneca reported Monday that its COVID-19 vaccine provided strong protection among adults of all ages in a long-anticipated U.S. study, a finding that could help rebuild public confidence in the shot around the world and move it a step closer to clearance in the U.S.
From The Associated Press